Successful testing and registration of an innovative drug requires years of research and millions of investments, but the little-known Russian company Himrar, which had never developed drugs at all before, suddenly managed to quickly and practically from scratch create and bring to the market drugs to combat COVID-19 ( favipiravir) and for the treatment of HIV patients (Elpida, aka elsulfavirin). Medicines from Khimrar, which has an office in the United States and connections with the Ministry of Industry and Trade, were registered very quickly, instantly got into clinical recommendations and the list of vital drugs, received state support, promotion and government purchases worth billions of rubles. At the same time, Elpida has not proven its effectiveness according to the required international standards. The reason for the unprecedented recognition of a dubious drug is very simple – the manufacturing company is directly connected with the official of the Ministry of Health Alexey Mazus.
The Insider has already told the story of the development and introduction to the Russian market of favipiravir, the ineffectiveness of which in COVID-19 has been proven by foreign studies. Another drug from the same manufacturer, Elpida (elsulfavirin), entered the market almost as quickly, is also not used in other countries and raised no less questions from patient organizations. At the same time, it costs more than the internationally recognized and thoroughly researched drug Tivicay (dolutegravir), instead of which it is purchased. In less than five years, the state has purchased Elpida for patients for about 7 billion rubles.
One of the explanations for the unprecedented success of the drug may be that Alexei Mazus, the chief specialist of the Russian Ministry of Health on HIV infection, is associated with its manufacturers.
Mazus does not believe in condom protection against HIV, denies the need for treatment of drug addicts, and since the 1990s has promoted the idea that mass forced HIV testing will save Russians from infection. This wise policy has led to the growth of the HIV epidemic in Russia, some regions of the country are among the world leaders in the proportion of the adult population affected by HIV infection, and only half of all patients are provided with the necessary drugs. But the more HIV carriers there are and the greater the shortage of drugs recognized by international standards, the more expensive and in large volumes one can sell one’s know-how, the Elpida miracle drug. Meanwhile, how well this drug works and how it is combined with other drugs is essentially unknown – the manufacturer managed to register Elpida, having only a couple of small clinical studies with a strange design. By recommending it, the Ministry of Health is essentially experimenting on the health of terminally ill patients while bringing money to the accounts of a friendly pharmaceutical business.
“Elpida” – a small miracle or a dummy
The history of Elpida’s manufacturer, Khimrar, was presented in the media in the mid-2000s as one of the clearest examples of the development of innovations and the attraction of foreign investment to Russia. At the end of 2004, she opened a research center in the field of preclinical drug development in Khimki near Moscow. The investor was the American fund Torrey Pines Investment (actually not so American, but more on that later). $ 5 million was invested in the center, he began to research new substances that could potentially become medicines. The customers of these studies were pharmaceutical companies – such a scheme is regularly used in the world, as it saves money for large pharmaceutical corporations. Dating in the Ministry of Industry and Trade helped the business to advance – the founders of the company for a long time were friends With Sergey Tsybwho in those years headed the department of the ministry responsible for pharmaceuticals.
However, the founders of the company dreamed of not just fulfilling other people’s orders for the synthesis and verification of molecules.
Immediately after its founding, Viriom entered into a contract with the Swiss F. Hoffman-La Roche Ltd to research several molecules that had been developed at La Roche since the early 2000s. The partners felt that the new molecules could be effective against chronic viral infections: HIV and hepatitis B and C. La Roche decided to stop research on them, as it focused on anticancer drugs. In Russia, where in 2009 there were already about half a million HIV-infected people (only official ones, with an established diagnosis), they became interested in promising molecules. At the same time, the stage at which the developments of the Swiss company were located was not even clinical trials. A year later, from the received package of molecules, Viriom chose the one that began to be tested as a cure for HIV. She received the working title VM-1500 and eventually became the very “Elpida”.
The project was supported by the Commission for Modernization and Technological Development under President Dmitry Medvedev. In 2011-2013 Viriom received 150 million rubles from the Ministry of Industry and Trade for the development and research of the drug. Already in 2013 the company statedthat elsulfavirin has a pronounced antiviral effect observed in patients with HIV. To the sharp question of a Kommersant journalist: “Is this really our long-awaited breakthrough in the most modern industry – pharmacology – and the challenge of the global AIDS epidemic in the world ?!” co-owner of Khimrar, university comrade Ivashchenko Mykola Savchuk replied:
This miracle, however, was poorly supported by clinical trials. According to open data from clinical trial registries, the company conducted only three human studies until 2013. The number of volunteers did not exceed a few dozen, almost all of them were healthy, and they checked the pharmacokinetics of the substance – how the substance is converted into others while in the body and how it is then excreted from the body. During a seven-day study in Thailand, 14 HIV-infected patients received the drug for seven days, after which the researchers came to conclusionthat the drug showed “optimal antiviral activity”. This was the only placebo-controlled trial of Elpida – that is, one group of patients was given a “dummy”, the other was given a new medicine. And the number of study participants was very small. “Such a sample is only suitable for a preliminary study, that is, to determine whether there is any signal in terms of effectiveness at all. Seven days is also not enough time to evaluate the effectiveness. Typical samples used to study effectiveness in HIV are several hundred people, ”the head of scientific expertise at the pharmaceutical venture fund Inbio Ventures told The Insider. Ilya Yasny. For example, the US Food and Drug Administration (FDA), which is authoritative for doctors all over the world notesthat several hundred people should participate in this phase of the study of the effectiveness of the drug. This is important not only to avoid statistical error, but also, in the case of HIV, to understand how the drug works in patients with different health conditions or virus mutations.
First study on a relatively large group started in Russia in 2014 and lasted almost two years. The scientists compared the effectiveness of Elpida (in a three-drug regimen) with efavirenz (Stokrin, along with two similar drugs). Efavirenz was then part of the first line of therapy (i.e., was preferred treatment if there are no contraindications) in developed countries, but patients often refused it due to a large number of side effects, including psychiatric ones (depression, nightmares, insomnia, confusion, and others). Authors of the Russian study declaredthat such side effects were less common in those who took Elpida than in those who took Stokrin. But there were no side effects, for example, with EpiVac, the medicine for COVID-19, simply because it was empty. Did Elpida manage to show any efficiency?
In short, the very design of the study was such that no conclusions could be drawn from it that Elpida was in any way effective. Let’s start with the methodology. In the published article, the authors refer to the design of the study as “multicenter, randomized, partially blind, direct comparison.” If translated from scientific language to ordinary, this means that the trials were carried out in several hospitals (multicenter), scientists and patients did not know who was participating in which part of the experiment (randomized), some participants in the experiment (most likely doctors and / or manufacturers) knew which participants were taking which medications (partially blind), the study compared multiple drugs (comparative), and expected that the new drug would improve patients’ quality of life (direct). In fact, the authors pointed out that a previous study on 14 patients already proves that the drug lowers the viral load well, and they were only interested in how much easier it is tolerated by patients than Stokrin.
In addition, modern GMP and GCP standards, good manufacturing and clinical practice are not fully observed in Russia. This can lead to the fact that manufactured drugs may be of poor quality, and research data — falsified in whole or in part. Ilya Yasny points out that the researchers changed the final criteria after end of the study. If at first they set out to test the activity of a drug for suppressing the virus, then at the end they indicated only a comparison of the activity with Stokrin, without even indicating how the effectiveness would be measured and how to determine which drug is better.
The authors of the trials state that both treatment regimens (with Elpida and with Stokrin) showed approximately the same effectiveness, but the presented study results cannot be considered reliable, Yasny points out, due to the fact that there are errors in the design of the study, the results are poorly described. . And even more so, in no case can a drug be considered worthy of a recommendation based on the result of one such study.
The Ministry of Health did not think so, and according to the results of this one study gave out “Elpide” licensed (and the regional health departments immediately began to purchase the medicine), and after a few months recommended include it in the first – that is, preferred – line of HIV therapy. The drug immediately began to buy AIDS centers throughout Russia. In fact, the drug had previously been studied in less than 200 people. The results of such studies by world standards are not reliable enough to start prescribing the drug to all patients in a row. Comparison of the efficacy and tolerability of Elpida with other drugs, except for efavirenz, has also not been carried out – probably because according to Russian clinical guidelinesand by WHO recommendations In those years, it was efavirenz that was the preferred first line of therapy.
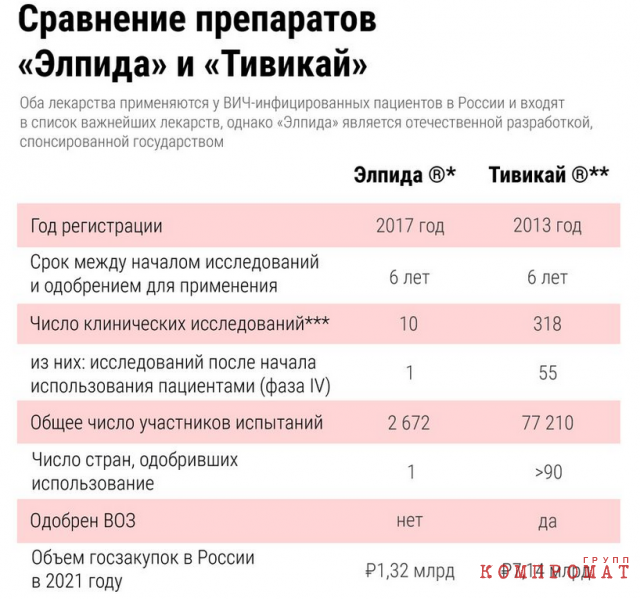

The noisy success of Elpida in Russia was not noticed outside of it, in 2019 WHO recommended include a completely different drug, dolutegravir (Tivicay), in the first line of therapy, as it turned out to be more tolerable and effective than other drugs in the same group (European AIDS Clinical Society (EACS) suggested do so in 2017).
Initially (in 2019), Elpida (elsulfavirin) appeared in project guidelines for HIV treatment in a rather modest role: the drug was recommended only as an alternative therapy for people who are not suitable for the most prescribed and preferred drug in the regimen for HIV patients, efavirenz. In addition, the first drafters of the recommendations at first honestly mentioned that Elpida should not be used in a number of cases: for example, in combination with the main drugs for tuberculosis – rifabutin and rifampicin, or with omeprazole (the main drug for gastritis), or with some female contraceptives (“Rigevidon”), or with the antibiotic clarithromycin – in a word, in combination with a number of fairly popular drugs.
However, already in 2020, Evgeny Voronin, the chief freelance HIV specialist of the Ministry of Health (who wrote these recommendations), was replaced by Alexander Mazus, who returned to this position, and immediately with his participation, new recommendations, in which Elpida not only ended up in the preferred treatment regimen, but all information about interactions with other drugs was removed. In fact, if the doctor followed these recommendations and did not read instruction to the drug (which describes dozens of drugs that cannot be used with Elpida, or can be used, but with great care), he could prescribe a regimen for the patient, which in the end would be harmful to health.
New recommendations, including Elpida in the first line of therapy, do not deny that the drug is almost not studied: the schemes with its participation are marked with a 4C level of reliability – according to the Ministry of Health, this stands for as “weak recommendation (lack of good quality evidence)”. However, in the end, nothing prevents advising to start therapy with this particular drug.
Cabal between officials and manufacturers
Simultaneously with the return of Mazus, the non-profit National Virological Association also became active. In 2021, the association received more than 19 million rubles in profit from commercial activities – this has not happened in the previous seven years of its history.
Honorary members of this organization include Anita Smith and William Shepard Smith Jr. Christian activists who have received US budget support to fight HIV in children since the days of President George W. Bush. Their funding from the government causes questions other organizations, as they stigmatize risk groups (homosexuals and drug users), oppose the use of condoms – in general, they do everything that Mazus and his colleague Lyudmila Stebenkova do in Russia (and for state money), although such practices are everywhere world showed inefficiency in the fight against HIV.
It is noteworthy that at least seven foreigners are listed on the website among the honorary members of the Mazus Association, but the organization has never reported on receiving funds, for example, membership fees, from foreign persons. But it is even more interesting that after the restart of the association’s website, a section appeared there listing the member companies of the organization. It turned out that over the years of their existence, there have been as many as two of them: Janssen (pharmaceutical division of Johnson & Johnson) and Viriom, the manufacturer of the same Elpida, which is categorically recommended by the association for appointment in the first line of therapy, despite the fact that this is one of the most expensive medicines available in Russia today. In fact, Mazus did not even hide the fact that, using his official position, he included in the recommendations for the whole country a drug from the company that financed his organization.
Thus, the drug, the effectiveness of which was not proven by sufficient clinical trials according to international standards, was included in the list of recommended prescriptions in the first place, in the list of essential drugs. All this time, he was patronized by officials of the Ministry of Industry and Trade, the “chief specialist” Aleksey Mazus, who receives a salary from the Ministry of Health, and large businessmen from the pharmaceutical industry. In 2022, the authorities purchased more than 55 thousand annual courses of the drug, spending 4.3 billion rubles. After Mazus returned to his position at the Ministry of Health, purchases of Elpida increased significantly – before that, it was bought for 8-11 thousand courses.
At the same time, patient organizations recorded a number of cases when, when taking Elpida, the viral load grew, that is, the drug did not suppress the reproduction of the virus. When the question about this was addressed to Viriom, the manufacturer notedthat the matter may be in certain mutations of the virus, in which it becomes resistant to the drug. The fact is that the appointment of drugs of the same class as Elpida (non-nucleoside reverse transcriptase inhibitor, NNRTI) requires testing for mutations that cause drug resistance. However, since this analysis is expensive (more than 20 thousand rubles), in Russia it is almost never done at public expense, told The Insider in one of the patient organizations. The patients themselves may not be aware of the need for such an analysis, and if they do, not everyone can afford it. The company claims that Elpida is better than other drugs, as it is effective in many mutations where other drugs do not work. However, Viriom backs up its statements not with clinical trials of the drug, but with studies conducted even before the sale of Himrar. analysis of the promising molecule RO-0335. How often patients have to end up canceling Elpida is hard to say – the Ministry of Health does not provide information about which drugs are often used by patients.
Questions to the manufacturer in patients also arise in connection with the rules for taking the drug. The fact is that antiviral therapy, as a rule, is very sensitive to the time of administration (you need to drink the medicine at the same hour every day) and to whether there was a recent meal. In the instructions for Elpida, these data were repeatedly changed, because of which patients could not understand how best to take the medicine so that it would definitely have an effect. Such manipulations caused a heated discussion of the “dampness” of the drug on the forums of HIV-infected people.
Who makes money on Elpida
Viriom’s birthday, April 30, 2009, strangely coincides with the emergence of yet another company. On the same day, a businessman Ilya Tsigelnitsky, an acquaintance of Alexei Mazus, the chief freelance specialist of the Ministry of Health of Russia on HIV infection, registered Medbiotest LLC in Moscow. After signing of the contract between Viriom and the Swiss F. Hoffman-La Roche Ltd, Medbiotest received 5% in the authorized capital of Viriom, and a little later, according to the Unified State Register of Legal Entities, the director “Anti-HIV-Press” Alexander Uskov (she later changed her last name to Mazus).
The friendship between Mazus and Himrar did not end there. In 2018, Mazus’ son Matvey was enrolled in the first year of the MGIMO paid master’s program for the Public-Private Partnership program. The training appears to have been successful, because a year later, Himrar transferred to him a third in its subsidiary Avivir, whose main portfolio since the beginning of the COVID-19 pandemic has been the reselling of Korean tests for antibodies to the virus. In less than three years of existence, the company has only directly sold tests to Russian hospitals for more than 150 million rubles.
After Viriom began to produce Elpida for Russian hospitals, its capital began to include representatives of large pharmaceutical manufacturers and people associated with the Ministry of Industry and Trade. In 2018, the shares were received by the Swiss company APICON AG associated with La Roche, Andrey Reus, a former official of the Ministry of Industry and Energy and the head of Oboronprom, who since 2012, like many in the Ministry of Industry and Trade, has become interested in the production of medicines.
Another person close to the Ministry of Industry and Trade, who at the same time became a co-owner of Viriom, is Evgeny Maksimov. This entrepreneur is the founder and head of the Center for Plastic and Endoscopic Surgery Natalia Manturova, the wife of one of the richest Russian ministers – Denis Manturov. Surprisingly, Maximov himself then told Vademecum that he did not I know of his appearance in the capital of Viriom, and then stopped answering calls. Then, in 2019, a 30% stake in Viriom was transferred to Pharmstandard by billionaire Viktor Kharitonin.
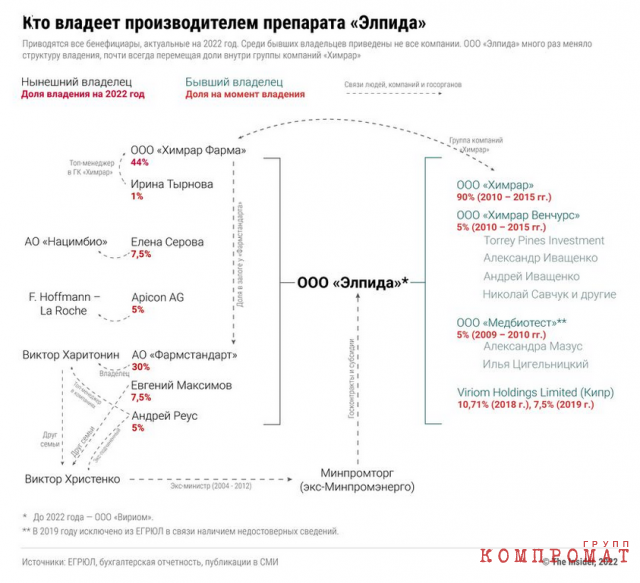
Thus, although Khimrar is engaged in the promotion of the Elpida drug and research on it, in fact, the production and profits from its sale under government contracts go mainly to the structures of Viktor Kharitonin, as well as people associated with the Ministry of Industry and Trade.
Self-invested from California: not-so-foreign investment
In the mid-2000s, Himrar was cited as an example as one of the high-tech companies attracting investments from abroad. As we remember, the American fund Torrey Pines Investment became the investor in the construction of the center in Khimki. In fact, this fund has always been associated with the founders of Khimrar – father and son Ivashchenko and Nikolai Savchuk and their American legal entity ChemDiv. That is, we can say that the attracted foreign investments were received from their own beneficiary.
The founders of ChemDiv began investing in construction in Russia in 2002 after Vladimir Putin approved document “Fundamentals of the policy of the Russian Federation in the field of science and technology development”. The Ministry of Industry and Trade was supposed to promote the emergence of technoparks in the country, but the construction of the center did without state investments – at that moment there was not even a mechanism for them. On the basis of the constructed center, scientists continued to create a collection of chemical compounds (chemists call them libraries) – in a few years, according to the founders of Himrar, their collection will be the largest in the world.
In fact, this means that the company’s chemists have created hundreds of thousands of different molecules and technologies for their synthesis – then such molecules can be tested for different “targets” to create drugs. Investments are needed for the next stage, but at that time biotechnologies were not yet popular in Russia. Andrey Ivashchenko, the founder of Khimrar, complained that private funds were not ready to invest in pharmaceuticals, and urged the state to do this:
The state heard these calls in 2011 as well. accepted federal target program “Pharma-2020” – “Khimrar” even took part in its development, and then received 13 contracts with the Ministry of Industry and Trade for a total amount of about 1.9 billion rubles. It was under this program that his daughter Viriom received investments for the development of Elpida, which actually remains one of two successful drugs in the Himrar portfolio. The second, Avifavir (favipiravir), we recall, was developed in the very first months of the COVID-19 epidemic under the auspices of the Russian Direct Investment Fund, the Ministry of Industry and Trade and the Innovation Promotion Fund. Like Elpida, the drug received a registration certificate and state contracts for hundreds of millions of rubles, practically without going through clinical trials.
Having registered Elpida and enlisting the support of top managers of the Ministry of Industry and Trade and Pharmstandard, the founders of Viriom decided to promote it in the United States. Nikolai Savchuk and other top managers associated with the American company ChemDiv founded a new company, Viriom Inc., in San Diego, where the ChemDiv office is located. Company claimswhich specializes in the development of antiviral drugs, but in fact its portfolio contains only Elpida and its combination with tenofovir and emtricitabine, registered in Russia and not undergoing reliable clinical trials, as well as the same favipiravir for the treatment of COVID-19.
On the website of the American Viriom Inc. not a single affiliation with the Russian “big brother” is mentioned. However, even the company’s logo matches the one that the Russian “Viriom” has used for years, presenting the results of its research at international conferences.
 Logos used by Russian and American Viriom in documents and websites
Logos used by Russian and American Viriom in documents and websitesAmerican company database Crunchbase indicates, which is on the board of directors of Viriom Inc. Aleksey Mazus also enters, and also that he is her adviser. The source of this information is unknown, but the only large-scale study of Elpida on infected volunteers in Russia was carried out just in the infectious diseases hospital No. 2 on Sokolina Gora in Moscow, where the Mazus AIDS Center is located.
In the summer of 2022, Viriom Inc. also added depulfavirin, an injectable anti-HIV drug for infrequent (once a month) use, to its portfolio. Although Viriom writes that it has already been approved for market launch, it is not known in Russia or in other countries. In July 2022, to promote the drug, a site, however, as of September 2022, there was no information on it. Judging by the description of the drug, we are talking about the substance VM-1500A-LAI (deselsufavirin), which was studied in Russia in 2019–2020. The results of the study of the pharmacokinetics of the drug in 2020 at a conference in Boston submitted Viriom employees and again Aleksey Mazus. In fact, this is the same elsulfavirin, but in a special injectable form for use no more than once a month. In April 2022, clinical trials began in ten Russian hospitals. study efficacy and safety of switching to a regimen with this drug in 505 HIV-infected volunteers. It will last for a year and a half. This study is already being conducted by the American company Viriom.

Thus, the managers of the Himrara business in the United States, including the promotion of Elpida as a global innovation, are Mykola Savchuk, as well as his American partner Ronald Demus, who has been working in the structures of Ivashchenko and Savchuk for 20 years. The manufacturer of Elpida (by the way, in 2022 the Russian Viriom was renamed Elpida LLC) and Avifavir has ties with Skolkovo, RDIF, the Ministry of Industry and Trade and other Russian organizations, which does not interfere with Savchuk in his profile in professional social LinkedIn networks condemn Russian authorities are for invading Ukraine – after all, he positions himself primarily as an American entrepreneur, investor and scientist.